The intermediates in a chemical reaction photographed ‘red-handed’
Researchers at the Materials Physics Center CSIC-UPV/EHU, the DIPC, and CIC nanoGUNE, in the framework of an international collaboration, have for the first time imaged and identified the bond configuration of the intermediates in a complex sequence of chemical transformations of enediyne molecules on a silver surface, thus resolving the microscopic mechanisms that account for their behaviour. This piece of research has
been published in the latest issue of the journal Nature Chemistry.
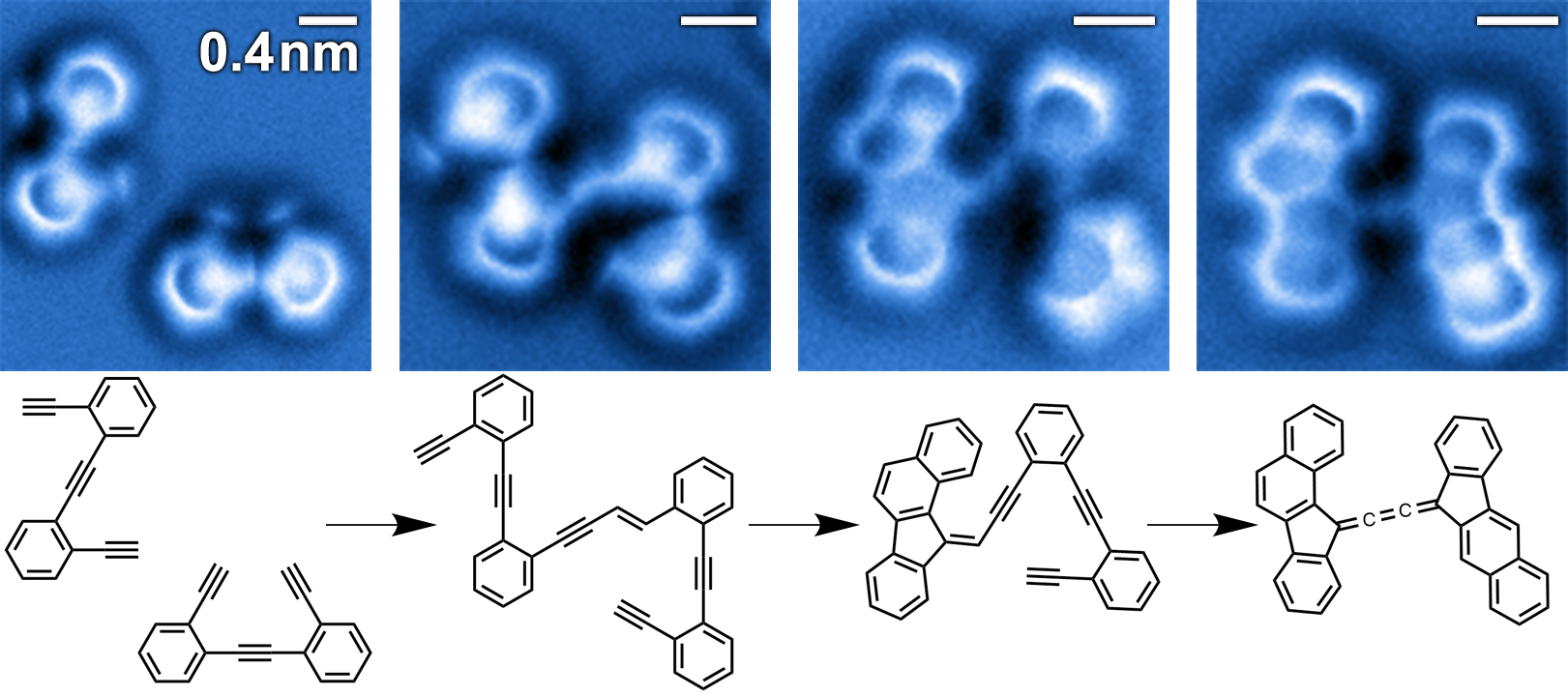
One of the long-standing goals being pursued by chemists has been to succeed in following and directly visualising how the structures of molecules change when they undergo complex chemical transformations.
Reaction intermediates, which are highly unstable substances that form in different steps in a reaction before the products are obtained, are particularly difficult to identify and characterise owing to their short lifetimes. Getting to know the structure of these intermediate species may be very helpful in understanding the reaction mechanisms and, what is more, could have a great impact on the chemical industry, materials science, nanotechnology, biology and medicine.
In the framework of this international collaboration, the bond configuration of the reactants, the intermediate and final products of a complex, organic reaction have been imaged and resolved at the single-molecule level. The prestigious journal Nature Chemistry has published this research in its latest issue.
The team has obtained the images of the chemical structures associated with different steps in the reaction cascade involving multiple steps of enediyne molecules on a silver surface, using non-contact atomic force microscopy (nc-AFM) with a particularly sensitive tip: it uses a very fine needle that can detect the smallest bumps on an atomic scale (in a way not unlike reading in Braille) as it absorbs a carbon monoxide molecule that acts like a “finger” on the text to increase its resolution.
Stabilizing the intermediates
By combining the latest advances in numerical calculus and the classical analytical models that describe the kinetics of sequential chemical reactions, an area that explores the speed of the reactions and the molecular events taking place in it has been proven. So to explain the stabilization of the intermediates, it is not enough just to consider their potential energy, it is essential to bear in mind the energy dissipation and the changes in molecular entropy, which measures how far a system is organised. The surface, and in particular the interaction of the extremely unstable intermediates with the surface, play a key role for both the entropy and the dissipation of energy, which highlights a fundamental difference between the surface-supported reactions and gas-phase or solution chemistry.
Additional information
The research has been carried out by various research groups led by Felix Fischer, Michael Crommie, and Angel Rubio (University of California at Berkeley, Lawrence Berkeley National Laboratory, EHU, and Max Planck
Institute for the Structure and Dynamics of Matter in Hamburg), and by Ikerbasque researchers Dimas Oteyza (CSIC/EHU and DIPC) and Miguel Moreno Ugeda (CIC nanoGUNE).
Bibliographical reference
A. Riss, A. Pérez Paz, S. Wickenburg, H.-Z. Tsai, D. G. de Oteyza, A. J. Bradley, M. M. Ugeda, P. Gorman, H. S. Jung, M. F. Crommie, A. Rubio and F. R. Fischer. “Imaging Single-Molecule Reaction Intermediates Stabilized by Surface Dissipation and Entropy”. Nature Chemistry. 2016. DOI: 10.1038/nchem.2506
