Related news by tag NANOBIOMECHANICS
The forces brought to bear on proteins: towards a new biology

The Granada-born chemist Raúl Pérez-Jiménez has come to nanoGUNE as an Ikerbasque Research Professor after having spent eight years at Columbia University in the City of New York. He has brought with him a promising line of research that is looking into how mechanical forces affect the proteins; it is something that is linked to a whole host of biological processes, including diseases like cancer, viral and bacterial infections, or myocardial infarction.
When we tug one end of a skein of tangled wool, it unravels, and if we go on tugging when the yarn is taut, it will break. The wool stretches and breaks as a result of a mechanical force. Something similar happens all the time in everything around us and in everything we are made of. From the Earth itself right down to the tiniest of nano-elements in our bodies, everything “functions”, at least partly, thanks to the action of mechanical forces. Yet little research has been done into how force affects the most basic of biological processes.
“Proteins subjected to mechanical forces are widely known to be linked to diseases yet, paradoxically, little is known in this respect. Firstly, because there were no techniques for studying these forces, and secondly, because traditionally they have been studied on a cell level with more or less established tests that did not take the mechanical component into consideration,” explained Pérez-Jiménez, leader of nanoGUNE’s new Nanobiomechanics Group. “The idea is to measure, and above all, to try and control the effect of the mechanical forces on proteins in order to generate fresh knowledge that could be used for acting on different diseases,” said Pérez-Jiménez.
Ancestral clues
One of the lines of research that Pérez-Jiménez has set up at nanoGUNE is focusing on the study of how proteins have evolved, from the origin of life up until the present day. It is a new scientific field which this Granada-born chemist has participated in right from the start. In actual fact, Pérez-Jiménez was one of the scientists in the group that resurrected proteins, which were over 4,000 million years old, in the laboratory; this piece of research was published in 2011 in the prestigious journal Nature Structural & Molecular Biology and it continues to bear fruit, as shown by another paper published in August of this year in the journal Structure. It is a journey back in time carried out by means of biocomputing techniques. Using modern protein sequences, scientists build phylogenetic relationships kinships from which it is possible to obtain the sequence of the ancestors. These ancestral proteins provide highly valuable information on the evolution of the protein structure and, as if that were not enough, they have unique properties that can help to understand their modern descendants better. The combination of biomechanics and the resurrecting of ancestral proteins has the potential to create new proteins that could be of tremendous use in medicine and biotechnology.
The challenge of mechano-pharmacology
The proteins resurrected by the researchers were thioredoxins, an enzyme that acts as an antioxidant and which can be found in all living organisms. Pérez-Jiménez knows this enzyme well and has set himself the challenge to go on tugging at the yarn. Thioredoxin is involved in various diseases, from a simple inflammation to AIDS, which makes it particularly interesting. In thioredoxin and in many other proteins important for our health, their function is altered due to the effect of mechanical forces. Taking this premise further, Dr. Pérez-Jiménez is starting a new line to study thioredoxin and other proteins affected by force. This new line has its sights on the challenge to be able to control the effect of mechanical forces by means of mechano-active molecules, which is known as mechano-pharmacology.
They have the most advanced equipment to launch the research in this field: two cutting-edge atomic force microscopes, unique in their class, and of the type that have never before existed in the country. These instruments enable very small forces to be applied, in the order of piconewtons, to the proteins. “A protein is a molecule comprising a linear chain of amino acids which in its resting state is folded up, similar to a spring or a skein of wool. What we do is place one end of the chain on a surface and the other on a point located in the microscope. Then we apply the desired force and tug at the protein. This process provides us with information that could not be obtained until very recently and which could be tremendously useful,” explained Pérez-Jimenez.
Today, medicine is becoming increasingly “nano” and the most innovative solutions are being sought and found on a nanoscale. “Diseases usually affect tissue, cells and finally molecules,” explained nanoGUNE’s new researcher, “and our aim is to start by studying the mechanical forces on a molecular level in order to discover new processes that could help to develop new drugs.” “I’m optimistic, but realistic at the same time, and I’m aware of the effort that all this involves. Our aim, ten years from now, is to be able to develop at nanoGUNE new techniques based on mechanical forces that could have an application in specific diseases,” concluded Pérez-Jiménez.
Raúl Pérez-Jiménez
Raúl Pérez-Jiménez (Granada, Spain, 1977) is an Ikerbasque Research Professor and the leader of nanoGUNE’s Nanobiomechanics group. He obtained a PhD in Chemistry from the University of Granada in 2005 and joined the lab of Prof Julio M. Fernandez at the University of Columbia (City of New York), where he spent eight years, firstly as a post-doctoral researcher and later as an assistant researcher. He came to nanoGUNE in February to set up a new line of work in the field of nanobiomechanics.
CIC nanoGUNE
The nanoGune Nanoscience Cooperative Research Centre, located in Donostia-San Sebastian (Basque Country), is a research centre set up with the mission to develop basic and applied research in nanoscience and nanotechnology; it does so by encouraging the high level capability and training of researchers in this field, and by promoting cooperation among the various agents of the Basque Science, Technology and Innovation Network (universities and R&D centres) and between these agents and industry.
Fundación Repsol Entrepreneurs Fund supports EVOLGENE project
Fundación Repsol Entrepreneurs Fund has the objective of promoting innovation and entrepreneurial development in the field of energy efficiency and new energy sources, making use of the opportunities for improvement offered by the energy sector. In its third edition, Fundación Repsol’s Entrepreneurs Fund has selected six innovative energy efficiency projects and four “Ideas” among 746 proposals. The judging panel emphasised the high degree of innovation of the selected proposals as well as their potential to become companies that with their novel technological solutions contribute to the business development and the creation of new jobs in the energy sector.
The EVOLGENE project, developed by nanoGUNE’s Nanobiomechanics Group Leader and Ikerbasque Research Professor Raul Perez Jimenez, is one of the four selected “Ideas”: proposals in the early state of development that will receive support to undertake a proof of concept. The project will receive business and technological mentoring as well as one-year financial support for the development of a business venture.
The paleoenzymology project uses bioinformatic resources and genomic data to reconstruct ancestral enzymes from billions of years back which are able to work under extreme temperature and pH conditions and have higher efficiency than current enzymes. These enzymes wil be used for the production of biofuel by the energy industry. Dr. Perez Jimenez, who will lead the work-team formed by Aitor Manteca and Nerea Barruetabeña, states that “the goal is to use enzymes from the past for the energy of the future”.
ACSnano: "Probing the Effect of Force on HIV-1 Receptor CD4"
The biophysicists work has also been highlighted by C&EN, where Katherine Bourzac explains the work in “Mechanical Force May Help HIV Invade Cells”:“A team of biophysicists has tested a hypothesis that HIV may exert force on cell-surface receptors as part of the chain of events that leads to infection.Through single-molecule experiments, the researchers demonstrated that a mechanical force causes conformational and chemical changes to the cellular protein targeted by HIV. This is the first study to examine how mechanical forces affect the physical and chemical properties of a cell receptor, which may one day lead to development of new kinds of treatments for disease, the researchers say.
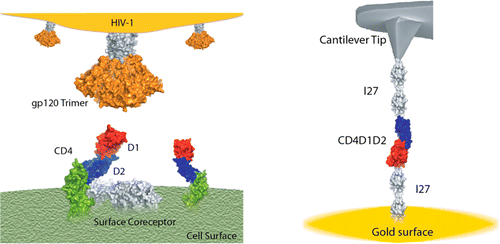
Perez-Jimenez spent five years performing experiments to look at the CD4 protein in isolation, simply asking whether mechanical forces have an effect on it. He worked with biologists to engineer cells to produce the part of the CD4 protein that interacts with gp120, connected to linker proteins on either side. They then tethered the linkers to a gold surface and used an atomic force microscope probe to pull on them while observing physical and chemical changes.
Under forces as low as 20 piconewtons, the CD4 protein elongates and becomes more flexible. Perez-Jimenez also saw that disulfide bonds normally tucked within the CD4 protein get exposed and become more reactive. This, he says, may support some scientists’ hypothesis that reactions with these disulfide bonds help HIV gain entry into T cells. These results together show that even very small mechanical forces might help exert conformational and chemical changes on the CD4 receptor that could help the virus infect cells.
Perez-Jimenez then tested whether the investigational drug ibalizumab, an antibody that binds to CD4 and prevents HIV infection, had a mechanical effect on the cell receptor. He found it took more force to pull on the CD4 protein when bound to ibalizumab, meaning that the antibody makes CD4 more rigid. The results suggest that mechanical effects may be part of the mechanism by which ibalzumab works, Perez-Jimenez says.
The next step is to figure out how much mechanical force HIV applies to CD4 in the membranes of living T cells—an experiment Perez-Jimenez is planning.
‘The HIV-CD4 interaction is highly dynamic and difficult to observe,” says Andrew Ward, a structural biologist who studies membrane proteins and HIV at Scripps Research Institute, La Jolla, Calif. Understanding the biophysics of how these interactions work is important, even if it’s not yet clear how to exploit those mechanical effects with a drug. The new data have shown the field “something that no other technique can reveal’, he says.”
