Research on biocatalytic processing of cellulose featured by Communications Chemistry
The publication entitled ‘Resurrection of efficient Precambrian endoglucanases for lignocellulosic biomass hydrolysis’ has been featured in a themed collection put together by Communications Chemistry that showcases a selection of the Structural Biology, Biocatalysis, and Bioconjugation Research published in the journal.
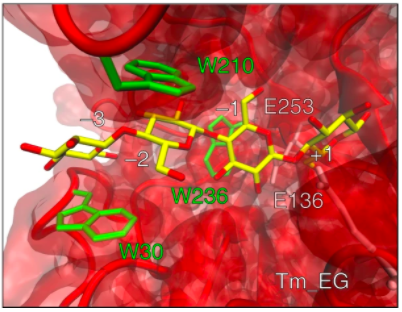
The article of the Nanobiotechnology group sheds some light on the efficient biocatalytic processing of cellulose, which is a longstanding goal in the biotechnology industry. In the publication by Barruetabeña et al, an ancient endogluconase is recreated using ancestral sequence reconstruction and the basis for its thermal stability and favourable catalytic properties are probed in the laboratory.
Figure: Structure and interaction of cellotetraose substrate bound to catalytically relevant residues in the active site of Tm_EG