ACSnano: "Probing the Effect of Force on HIV-1 Receptor CD4"
NanoGUNE’s Nanobiomechanics Group, led by Ikerbasque Professor Raul Perez-Jimenez, in collaboration with the Department of Biological Sciences of the Columbia University (US) and the Aaron Diamond AIDS Research Center (US), has tested that mechanical force may help HIV to invade cells. The research has been recently published in ACS Nano (DOI: 10.1021/nn503557w)
The biophysicists work has also been highlighted by C&EN, where Katherine Bourzac explains the work in “Mechanical Force May Help HIV Invade Cells”:“A team of biophysicists has tested a hypothesis that HIV may exert force on cell-surface receptors as part of the chain of events that leads to infection.Through single-molecule experiments, the researchers demonstrated that a mechanical force causes conformational and chemical changes to the cellular protein targeted by HIV. This is the first study to examine how mechanical forces affect the physical and chemical properties of a cell receptor, which may one day lead to development of new kinds of treatments for disease, the researchers say.
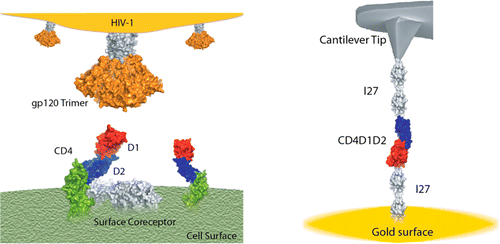
HIV infection starts when a glycoprotein on the surface of the virus, gp120, binds with the CD4 receptor on the surface of a T cell. The binding triggers a cascade of other protein-protein interactions, which eventually allows the virus to fuse its membrane to the cell’s own. But the details of the initial interaction between CD4 and gp120 are still not known, says Raul Perez-Jimenez, a chemist who specializes in nanoscale biomechanics at CIC nanoGUNE, a nanoscience research center in San Sebastian, Spain. In the past, Perez-Jimenez has used a method called force spectrometry to study the mechanical properties of the muscle protein myosin. He wondered whether the gp120-CD4 binding might have a mechanical component, too.
Perez-Jimenez spent five years performing experiments to look at the CD4 protein in isolation, simply asking whether mechanical forces have an effect on it. He worked with biologists to engineer cells to produce the part of the CD4 protein that interacts with gp120, connected to linker proteins on either side. They then tethered the linkers to a gold surface and used an atomic force microscope probe to pull on them while observing physical and chemical changes.
Under forces as low as 20 piconewtons, the CD4 protein elongates and becomes more flexible. Perez-Jimenez also saw that disulfide bonds normally tucked within the CD4 protein get exposed and become more reactive. This, he says, may support some scientists’ hypothesis that reactions with these disulfide bonds help HIV gain entry into T cells. These results together show that even very small mechanical forces might help exert conformational and chemical changes on the CD4 receptor that could help the virus infect cells.
Perez-Jimenez then tested whether the investigational drug ibalizumab, an antibody that binds to CD4 and prevents HIV infection, had a mechanical effect on the cell receptor. He found it took more force to pull on the CD4 protein when bound to ibalizumab, meaning that the antibody makes CD4 more rigid. The results suggest that mechanical effects may be part of the mechanism by which ibalzumab works, Perez-Jimenez says.
The next step is to figure out how much mechanical force HIV applies to CD4 in the membranes of living T cells—an experiment Perez-Jimenez is planning.
‘The HIV-CD4 interaction is highly dynamic and difficult to observe,” says Andrew Ward, a structural biologist who studies membrane proteins and HIV at Scripps Research Institute, La Jolla, Calif. Understanding the biophysics of how these interactions work is important, even if it’s not yet clear how to exploit those mechanical effects with a drug. The new data have shown the field “something that no other technique can reveal’, he says.”
