Related news by tag Nanobiotechnology
CIC nanoGUNE expands its patent portfolio
The diversity of application fields demonstrates the great versatility of nanoscience research, and the increase in licensed methods and technologies demonstrates its ability to improve and add value to industrial processes and products. "Our goal is to continue doing research of excellence and transferring those specific developments with the potential to improve the competitiveness of companies to the economic sector," explained Ainara Garcia-Gallastegui, head of Technology Transfer at nanoGUNE.
Students from UPV / EHU, Tecnun, UAB and the UB carry out summer internships at CIC nanoGUNE
On Tuesday, 19 June, we welcomed a group of internship students that will carry out a research project at NanoGUNE during the summer. The director of the center, Jose M. Pitarke, received the students with a presentation talk about nanoGUNE, that was also attended by the researchers that will conduct the students’ projects.

The 11 students come from different universities, among which are, Unibersity of the Basque Country (UPV /EHU), Tecnun, Univeristy of Barcelona (UB), Autonomous University of Barcelona (UAB). This program offers to the students a real experience of work in a research laboratory in order to make it easier for them to take decision about their future professional life.
The students will collaborate and learn with the different research groups at nanoGUNE, such as nanooptics, nanodevices or nanomagnetism. They will carry out a research project for two months following the instructions of a researcher of their group.
Some of them started the internships at the beginning of the month and they have been very involved in the group’s work. "The truth is that we started very suddenly; the very first day they took me to the laboratory," says Amaia Ochandorena, a student of Biochemistry and Molecular Biology at the UPV/EHU.
All the students knew CIC nanoGUNE and stressed that "it is an important research center" and "offers and works with topics of much interest".
For these students, and also for undergraduate students of general, nanoGUNE offers the possibility of collaborating with the center for the completion of final graduate or master thesis projects, for which also opens a call for grants every year.
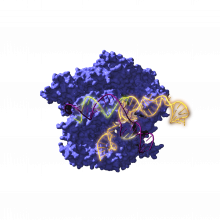
2.6 billion-year-old ancestors of the CRISPR gene-editing tool are resurrected
The project, led by Ikerbasque research professor Rául Pérez-Jiménez of CIC nanoGUNE, involves teams from the Spanish National Research Council, the University of Alicante, the Rare Diseases Networking Biomedical Research Center (CIBERER), the Ramón y Cajal Hospital-IRYCIS and other national and international institutions.
The first loudspeaker with graphene-based technology is already on the market

This work has emerged out of the urge to innovate of both nanoGUNE and SEAS; “the idea came about through our own interest and due to our ongoing quest for applications for the technologies and materials developed at nanoGUNE. There were papers published referring to the use of graphene oxide on metal surfaces exposed to hostile environmental conditions, and we came up with the idea of applying this to loudspeaker cones. SEAS expressed great interest in this idea from the start," said Pérez-Jiménez.
That is how the collaboration work between SEAS engineers and nanoGUNE researchers came about and which has produced results following numerous tests. “The first premium-range loudspeakers whose cones have a surface of graphene oxide are now on the market. It is a material which makes them longer-lasting without affecting the mechanical properties of the loudspeakers and even enhances them,” he pointed out. “The loudspeakers were tried out in situ in very damp locations where corrosion frequently occurs in equipment of this type, such as Singapore and Donostia-San Sebastian, and the results were hugely positive," said the nanoGUNE researcher.
nanoGUNE launches a new summer internship call for university students

Through this programme the Basque nanoscience research centre will this summer be receiving about ten new students in their 3rd and 4th years of Physics, Chemistry, Biology and Engineering. For a period of six weeks or two months the young students will be collaborating with nanoGUNE researchers in their research projects on subjects such as electron/spin phenomena and magnetism, nanoscale optics, nanoscale materials and nanobioengineering, among others.
To participate in the summer internship programme any students who are interested will need to submit their applications online via the nanoGUNE website, the deadline being 16 February. Full information relating to the call is available via the nanoGUNE website (www.nanogune.eu)
Basque research and technology, committed to the health alert of the COVID-19
The entities are working on the production of diagnostic tests, breathing devices, work protocols and artificial intelligence solutions to guarantee work safety, among other projects. Rikardo Bueno, general manager of BRTA, states that "from the very beginning, BRTA centres have shown their willingness to put their knowledge and creativity at the service of our society in response to COVID-19".
CIC nanoGUNE and CIKAUTXO join forces to optimize rubber
CIC nanoGUNE receives 1.5 million euros from the European Commission

It is also worth highlighting that the European FET Open program is a highly competitive call given that out of the 902 projects submitted only 58 (6.6%) have been funded in the whole of Europe.
Funding for these three projects exceeds 1.5 million euros, of which €747,000 corresponds to the bioUPGRADE project, in which Raúl Pérez-Jimenez is participating; €390,625 is for the INTERFAST project, in which Luis Hueso is collaborating; and €375,000 goes to the SINFONIA project, in which Luis Hueso is also participating.
The nanoGUNE researchers assert that “the SINFONIA and INTERFAST projects are enabling us to go on exploring the properties of organic materials in the field of the most competitive electronics, and to bring our basic research proposals closer to commercial devices with the help of top companies”. Likewise, the BioUPGRADE project “enables biotechnology to be used to transform natural resources into a new generation of “high-tech” biomaterials”.
SINFONIA
The main aim of the SINFONIA project (Selectively activated INFOrmation technology by hybrid Organic Interfaces), coordinated by Luis Hueso, is to develop a technology that will allow information to be stored and transported on a nanometric scale operating in the ultra-rapid THz regime, beyond current conventional processors. SINFONIA is proposing a completely new approach to information technology.
BioUPGRADE
The BioUPGRADE (Biocatalytic upgrading of natural biopolymers for reassembly as multipurpose materials) project, coordinated by Raúl Pérez-Jiménez, brings together advances in genomics, protein engineering and materials science for the purpose of transforming the principal polymers in nature into high-performance biomaterials with numerous applications, ranging from energy to medicine.
INTERFAST
The INTERFAST (Gated INTERfaces for FAST information processing) project, coordinated by Luis Hueso, will be developing a novel technology platform to control the magnetic response of a material by means of electric pulses and thus propose new forms of ultra-dense information storage.
The forces brought to bear on proteins: towards a new biology

The Granada-born chemist Raúl Pérez-Jiménez has come to nanoGUNE as an Ikerbasque Research Professor after having spent eight years at Columbia University in the City of New York. He has brought with him a promising line of research that is looking into how mechanical forces affect the proteins; it is something that is linked to a whole host of biological processes, including diseases like cancer, viral and bacterial infections, or myocardial infarction.
When we tug one end of a skein of tangled wool, it unravels, and if we go on tugging when the yarn is taut, it will break. The wool stretches and breaks as a result of a mechanical force. Something similar happens all the time in everything around us and in everything we are made of. From the Earth itself right down to the tiniest of nano-elements in our bodies, everything “functions”, at least partly, thanks to the action of mechanical forces. Yet little research has been done into how force affects the most basic of biological processes.
“Proteins subjected to mechanical forces are widely known to be linked to diseases yet, paradoxically, little is known in this respect. Firstly, because there were no techniques for studying these forces, and secondly, because traditionally they have been studied on a cell level with more or less established tests that did not take the mechanical component into consideration,” explained Pérez-Jiménez, leader of nanoGUNE’s new Nanobiomechanics Group. “The idea is to measure, and above all, to try and control the effect of the mechanical forces on proteins in order to generate fresh knowledge that could be used for acting on different diseases,” said Pérez-Jiménez.
Ancestral clues
One of the lines of research that Pérez-Jiménez has set up at nanoGUNE is focusing on the study of how proteins have evolved, from the origin of life up until the present day. It is a new scientific field which this Granada-born chemist has participated in right from the start. In actual fact, Pérez-Jiménez was one of the scientists in the group that resurrected proteins, which were over 4,000 million years old, in the laboratory; this piece of research was published in 2011 in the prestigious journal Nature Structural & Molecular Biology and it continues to bear fruit, as shown by another paper published in August of this year in the journal Structure. It is a journey back in time carried out by means of biocomputing techniques. Using modern protein sequences, scientists build phylogenetic relationships kinships from which it is possible to obtain the sequence of the ancestors. These ancestral proteins provide highly valuable information on the evolution of the protein structure and, as if that were not enough, they have unique properties that can help to understand their modern descendants better. The combination of biomechanics and the resurrecting of ancestral proteins has the potential to create new proteins that could be of tremendous use in medicine and biotechnology.
The challenge of mechano-pharmacology
The proteins resurrected by the researchers were thioredoxins, an enzyme that acts as an antioxidant and which can be found in all living organisms. Pérez-Jiménez knows this enzyme well and has set himself the challenge to go on tugging at the yarn. Thioredoxin is involved in various diseases, from a simple inflammation to AIDS, which makes it particularly interesting. In thioredoxin and in many other proteins important for our health, their function is altered due to the effect of mechanical forces. Taking this premise further, Dr. Pérez-Jiménez is starting a new line to study thioredoxin and other proteins affected by force. This new line has its sights on the challenge to be able to control the effect of mechanical forces by means of mechano-active molecules, which is known as mechano-pharmacology.
They have the most advanced equipment to launch the research in this field: two cutting-edge atomic force microscopes, unique in their class, and of the type that have never before existed in the country. These instruments enable very small forces to be applied, in the order of piconewtons, to the proteins. “A protein is a molecule comprising a linear chain of amino acids which in its resting state is folded up, similar to a spring or a skein of wool. What we do is place one end of the chain on a surface and the other on a point located in the microscope. Then we apply the desired force and tug at the protein. This process provides us with information that could not be obtained until very recently and which could be tremendously useful,” explained Pérez-Jimenez.
Today, medicine is becoming increasingly “nano” and the most innovative solutions are being sought and found on a nanoscale. “Diseases usually affect tissue, cells and finally molecules,” explained nanoGUNE’s new researcher, “and our aim is to start by studying the mechanical forces on a molecular level in order to discover new processes that could help to develop new drugs.” “I’m optimistic, but realistic at the same time, and I’m aware of the effort that all this involves. Our aim, ten years from now, is to be able to develop at nanoGUNE new techniques based on mechanical forces that could have an application in specific diseases,” concluded Pérez-Jiménez.
Raúl Pérez-Jiménez
Raúl Pérez-Jiménez (Granada, Spain, 1977) is an Ikerbasque Research Professor and the leader of nanoGUNE’s Nanobiomechanics group. He obtained a PhD in Chemistry from the University of Granada in 2005 and joined the lab of Prof Julio M. Fernandez at the University of Columbia (City of New York), where he spent eight years, firstly as a post-doctoral researcher and later as an assistant researcher. He came to nanoGUNE in February to set up a new line of work in the field of nanobiomechanics.
CIC nanoGUNE
The nanoGune Nanoscience Cooperative Research Centre, located in Donostia-San Sebastian (Basque Country), is a research centre set up with the mission to develop basic and applied research in nanoscience and nanotechnology; it does so by encouraging the high level capability and training of researchers in this field, and by promoting cooperation among the various agents of the Basque Science, Technology and Innovation Network (universities and R&D centres) and between these agents and industry.
Fundación Repsol Entrepreneurs Fund supports EVOLGENE project
Fundación Repsol Entrepreneurs Fund has the objective of promoting innovation and entrepreneurial development in the field of energy efficiency and new energy sources, making use of the opportunities for improvement offered by the energy sector. In its third edition, Fundación Repsol’s Entrepreneurs Fund has selected six innovative energy efficiency projects and four “Ideas” among 746 proposals. The judging panel emphasised the high degree of innovation of the selected proposals as well as their potential to become companies that with their novel technological solutions contribute to the business development and the creation of new jobs in the energy sector.
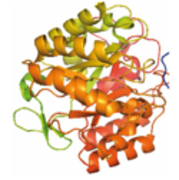
The EVOLGENE project, developed by nanoGUNE’s Nanobiomechanics Group Leader and Ikerbasque Research Professor Raul Perez Jimenez, is one of the four selected “Ideas”: proposals in the early state of development that will receive support to undertake a proof of concept. The project will receive business and technological mentoring as well as one-year financial support for the development of a business venture.
The paleoenzymology project uses bioinformatic resources and genomic data to reconstruct ancestral enzymes from billions of years back which are able to work under extreme temperature and pH conditions and have higher efficiency than current enzymes. These enzymes wil be used for the production of biofuel by the energy industry. Dr. Perez Jimenez, who will lead the work-team formed by Aitor Manteca and Nerea Barruetabeña, states that “the goal is to use enzymes from the past for the energy of the future”.
nanoGUNE Scholarship: call for Master Thesis students

The Scholarships will be of a total amount of 3,000€. This amount is for the whole period and will not be compatible with any other grant or funding awarded for the same purpose. Candidates have to be pre-registered and accepted at the above mentioned Master degrees in order to be eligible for these grants. Interested candidates can find all the information about the offered master projects and the application process following this link.
Besides the grants, nanoGUNE offers Master students coming from any official Master degree the possibility to develop their Master Thesis within one of its research groups.
Molecular toxicity of nanomaterials
Simon Poly has been developing his thesis project during four years at nanoGUNE, under de supervision of Felix Goñi, professor of the University of the Basque Country. His research work achieved the maximum qualification (cum laude) after the defense and assessment of his work by an international committee that included leading researchers in the field of nanobiology.
The thesis describes how the effect of nanomaterials on protein structure stability is due to the charge present at the surface of nanomaterials. Poly shows that different proteins will react differently with the same charge at the surface of nanomaterials. Interestingly, it is demonstrated that the effect of nanomaterials on protein structure was dependent not only on the charge present at the surface of nanomaterials but more importantly on the distribution of charge at the surface. On the basis of these data, the researchers were able to develop a new type of therapeutic agent based on controlled charge distribution at the surface of nanomaterials allowing specific interaction of nanomaterials and target protein without the need for coupled recognition molecules. This new agent is able to specifically interact with pathological proteins and aggregate them in a safe way in order to ease their removal from living organisms. Furthermore this discovery opens the way to a better understanding of nanomaterial toxicity and to new nanomaterial with increased in vivo stability for biomedical applications e.g. drug-delivery, biomedical implants, and tagging agents.
An international committee including leading researchers in the field was selected by the UPV/EHU to assess the research project:
- Jose Luis Pedraz Muñoz (UPV/EHU, Spain)
- Jesus Perez Gil (Universidad Complutense de Madrid, Spain)
- Shlomo Margel (Bar-Ilan University, Israel)
- Iseult Lynch (UCD, Ireland)
- Jose Maria Pitarke De la Torre (UPV/EHU, Spain)
The defense consisted of a presentation by the candidate of the main aspects of the research project followed by a long discussion about the questions that each one of the members of the committee raised around the research works that have been carried out during the whole PhD period. After its final deliberation, the committee decided to award the candidate the Doctor Degree with the highest mention existing at the Spanish University apto cum laude.
Raul Perez-Jimenez to receive the Enrique Pérez-Payá Prize

The Spanish Biophysical Society’s Enrique Pérez-Payá Prize was set up to recognise work in the field of Biophysics of a scientist under 40 years of age who develops his/her activity in Spain. The €1,500-prize is sponsored by BCN Peptides and Prima-Derm, and contributions from any of the fields of Biophysics are considered.
The awarding of the prize requires the winner to deliver a lecture at the 5th International Iberian Biophysics Congress due to be held in Oporto from 15 to 17 June. Raul Perez-Jimenez will be introduced by the Chairman of the SBE (Spanish Biophysical Society). A commemorative plaque and the prize will be awarded at the end of the congress.
ACSnano: "Probing the Effect of Force on HIV-1 Receptor CD4"
The biophysicists work has also been highlighted by C&EN, where Katherine Bourzac explains the work in “Mechanical Force May Help HIV Invade Cells”:“A team of biophysicists has tested a hypothesis that HIV may exert force on cell-surface receptors as part of the chain of events that leads to infection.Through single-molecule experiments, the researchers demonstrated that a mechanical force causes conformational and chemical changes to the cellular protein targeted by HIV. This is the first study to examine how mechanical forces affect the physical and chemical properties of a cell receptor, which may one day lead to development of new kinds of treatments for disease, the researchers say.
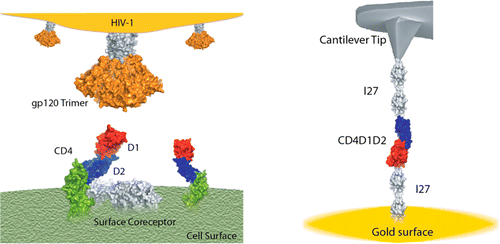
Perez-Jimenez spent five years performing experiments to look at the CD4 protein in isolation, simply asking whether mechanical forces have an effect on it. He worked with biologists to engineer cells to produce the part of the CD4 protein that interacts with gp120, connected to linker proteins on either side. They then tethered the linkers to a gold surface and used an atomic force microscope probe to pull on them while observing physical and chemical changes.
Under forces as low as 20 piconewtons, the CD4 protein elongates and becomes more flexible. Perez-Jimenez also saw that disulfide bonds normally tucked within the CD4 protein get exposed and become more reactive. This, he says, may support some scientists’ hypothesis that reactions with these disulfide bonds help HIV gain entry into T cells. These results together show that even very small mechanical forces might help exert conformational and chemical changes on the CD4 receptor that could help the virus infect cells.
Perez-Jimenez then tested whether the investigational drug ibalizumab, an antibody that binds to CD4 and prevents HIV infection, had a mechanical effect on the cell receptor. He found it took more force to pull on the CD4 protein when bound to ibalizumab, meaning that the antibody makes CD4 more rigid. The results suggest that mechanical effects may be part of the mechanism by which ibalzumab works, Perez-Jimenez says.
The next step is to figure out how much mechanical force HIV applies to CD4 in the membranes of living T cells—an experiment Perez-Jimenez is planning.
‘The HIV-CD4 interaction is highly dynamic and difficult to observe,” says Andrew Ward, a structural biologist who studies membrane proteins and HIV at Scripps Research Institute, La Jolla, Calif. Understanding the biophysics of how these interactions work is important, even if it’s not yet clear how to exploit those mechanical effects with a drug. The new data have shown the field “something that no other technique can reveal’, he says.”
New step towards clean energy production from enzymes
Fossil fuels supply over 80% of the world’s energy. Since the energy crises in the 1970’s and then in the 90’s, when concerns appeared about greenhouse effects, the search for alternative energy sources has been ongoing. Hydrogen has been a particularly popular candidate because its combustion only produces water. Biotechnology is uniquely poised for providing a means for using hydrogen as a source of clean energy. One possibility is using enzymes called hydrogenases that naturally occur in various microorganisms that live in anaerobic ecosystems, such as some bacteria living in soil and in the intestinal tract of animals, or unicellular algae.
Hydrogenases catalyze the conversion of protons in hydrogen molecules (H2), whose combustion releases energy that can be utilized for example in fuel cells and therefore be part of biotechnological devices. The active site that catalyzes this reaction contains metallic ions (Iron or both Iron and Nickel). The Iron-only variety of hydrogenases is the most active for the production of hydrogen molecules. Their remarkably complex active site –the so-called H-cluster– is buried within the core of a large protein. A fatal problem for being able to exploit hydrogenases in biotechnological applications is that when brought to the aerobic conditions of a bioreactor (under normal oxygen pressures), molecular oxygen degrades their active site. Understanding the mechanism of the degradation process of the H-cluster is therefore essential to design a hydrogen-based fuel cell, but studies so far had not been conclusive.
To solve this conundrum, an international team of researchers has combined experiments, molecular simulations, and theoretical calculations. Using electrochemical methods, they have precisely measured the rates of the different reaction steps involved in the degradation of the enzyme by oxygen. They have studied the dependence of these rates on experimental parameters like the electrode potential, pH, H2O/D2O exchange, and mutation of specific amino acids in the protein. These results confirm predictions from theoretical calculations. On one hand molecular dynamics simulations, conducted by nanoGUNE’s Ikerbasque Fellow David de Sancho, show the tunnels that oxygen follows to reach the active site of the protein, a necessary step for the degradation and to identify possible hot-spots for blocking these access tunnels. On the other, density functional theory has been used to elucidate the reaction products and evaluate the rate constants for the individual reaction steps.
The study published on August 22nd in Nature Chemistry has allowed to characterize unambiguously the complex reactions that occur in these large biological macromolecules using a highly innovative combination of computational and experimental approaches. “Although important challenges remain ahead for industrial applications, this study opens new avenues to efficiently exploit enzymes from living systems for clean energy production”, says De Sancho.
Representation of the diffusion of the gas molecule towards the active site of the Fe-Fe hydrogenase enzyme.
CIC nanoGUNE is awarded a project to investigate amyotrophic lateral sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS) is an incurable, neurodegenerative disease associated with mutations in several genes. Its effects are devastating and deprive patients of their mobility, and therefore of an acceptable quality of life, rapidly and progressively with a fatal outcome. This is why the strategy for modifying the genes that cause ALS undoubtedly offers one of the best hopes for treating this disease. The CRISPR gene editing technology provides tools for such a strategy.
The size of animals dating back 100-350 million years ago inferred from resurrected proteins
The Ikerbasque researcher Raúl Pérez-Jiménez of nanoGUNE’s Nanobiomechanics group has led a piece of research in which, starting from the sequences of the titin protein of a selection of modern day animals, they inferred the phylogenetic tree of tetrapods (all animals with four limbs including mammals, birds, reptiles and amphibians), and reconstructed the sequence that this protein would have had in the common ancestors of these animal groups. After obtaining their sequences, they synthesised a part of the ancestral proteins and studied their mechanical and chemical properties in the laboratory. This has enabled them to find a link between the properties of the protein and the body mass of the animals, which they have been able to confirm in the fossil record of each epoch. The scientific journal Nature Structural & Molecular Biology has just published the results of the research carried out by the nanoGUNE researchers in collaboration with the CNIC.

For this research, they selected over thirty animals from different taxonomic groups and of different sizes. “The complete genome of many animals was already available, so the first thing we did was to build a phylogenetic tree with the titin sequences of around thirty tetrapods. This tree enabled us to calculate the most probable sequences of the protein titin of four common ancestors of the taxonomic groups to which these animals belong: placental mammals, dating back about 100 million years; all mammals, dating back 170-180 million years; the common ancestor of the sauropsids, including all birds, reptiles and also dinosaurs, and which lived about 280 million years ago; and the common ancestor of all the animals we have studied, which would be the common ancestor of the tetrapods, dating back about 350 million years," specified Pérez-Jiménez.
Once they had the sequences, they synthesised the most elastic fragment of the proteins in the laboratory, and using an atomic force microscope available at nanoGUNE they were able to measure the mechanical resistance of each of the proteins. This instrument “allows one, literally, to take a protein and stretch it, unfold it mechanically using force, which is something similar to what happens to titin in the muscle,” remarked the researcher. That way they were able to compare the resistance or stability of all the titins being studied. In this study “we realised that the mechanochemical stability of the proteins depended on the number of disulphide bridges displayed by the titin, which are sulphur-sulphur bonds between two cysteine residues.
They were able to see that “the ancestral proteins were more resistant than those of today’s animals, and they had more disulfide bridges than the modern ones. Yet this difference was not so big compared with a small animal such as a finch”. This fact led them to think that there could be a link between the mechanochemical properties of titin and the size of the animals. “We saw a pretty good correlation: the larger animals had less stable proteins and the smaller ones more stable proteins. And that enabled us to predict the size of the ancestral animals”.
Once they had inferred the size of the common ancestors, the group led by Pérez-Jiménez compared them with fossil records and the scientific literature available in this respect. “We were able to see that there was substantial agreement; the ancestors of mammals, birds and tetrapods in general were really small, weighing less than 100 g; although we do of course have a margin of error inherent in the techniques themselves. This may not be surprising because one could consider that it is information that was already known, but what is new here is that we did not use a fossil, but started from a reconstructed protein, a purely molecular piece of information,” pointed out the researcher.
Pérez-Jiménez believes that “the interesting thing is that we have seen that mechanochemical evolution, how the titin gradually changed throughout evolution, and we have been able to reconstruct it”. These results will lead to pursuing the research further. “We would like to see, for example, whether this correlation with size is truly global, whether it exists in all animal groups,” he concluded.